Fe2O3 ironIII oxide Fe2O3 ferric oxide. For example Cu is called CopperI and Cu 2 is called CopperII in the names of compounds containing these ions.

Naming Ionic Compounds A Guided Inquiry Exercise
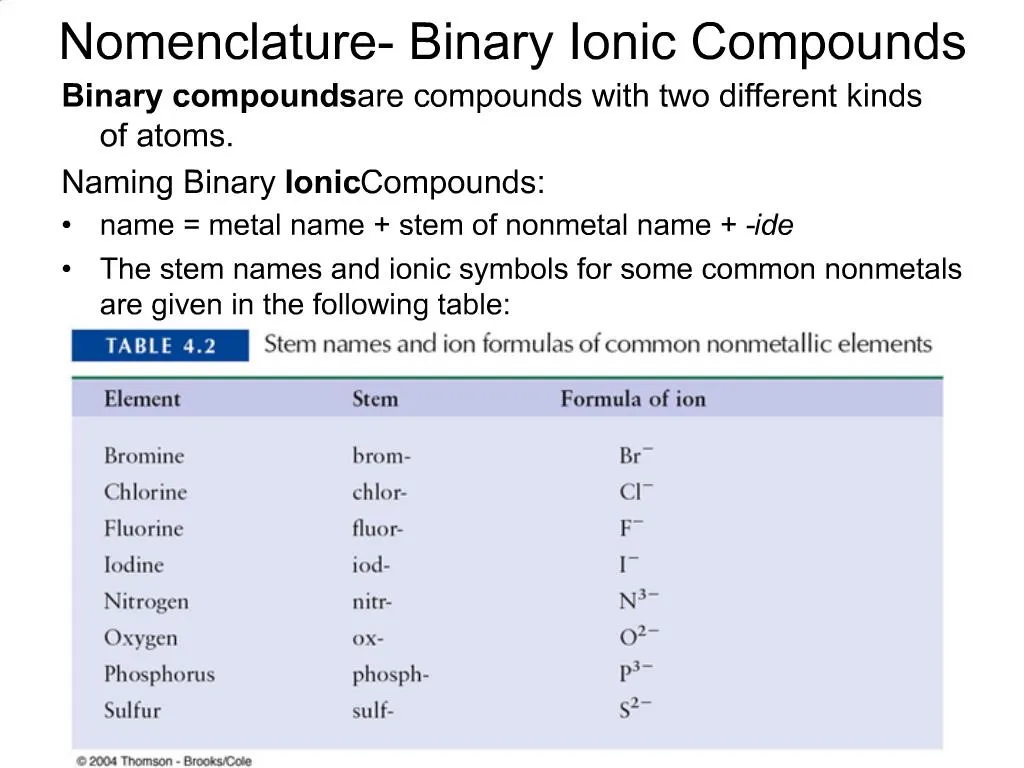
If the negative ion is a NON-METAL element àchange its ending suffix to ide.

. The naming of ionic compounds is dependent upon the type of ionic molecule formed from alkali metals alkaline earth metals or transition metals. These elements include astatine fluorine chlorine iodine and bromide. For example CO is carbon monoxide.
Binary Metal to Non-Metal. Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second. Keys to Naming Binary Ionic Compounds.
Al2O3 aluminum oxide MgCl2 magnesium chloride. Prefixes should not be used to indicate how many of each element is present. V 1 4 ft j t - l 1 tv.
By using an inquiry approach students analyze patterns and create their own rules helping them to not only remember the rules better but also to have a deeper understanding of the way compounds are named. For the non-metal the anion write the name on the Periodic Table and then replace the ending with ide. I E e Naming Ionic and Covalent Compounds.
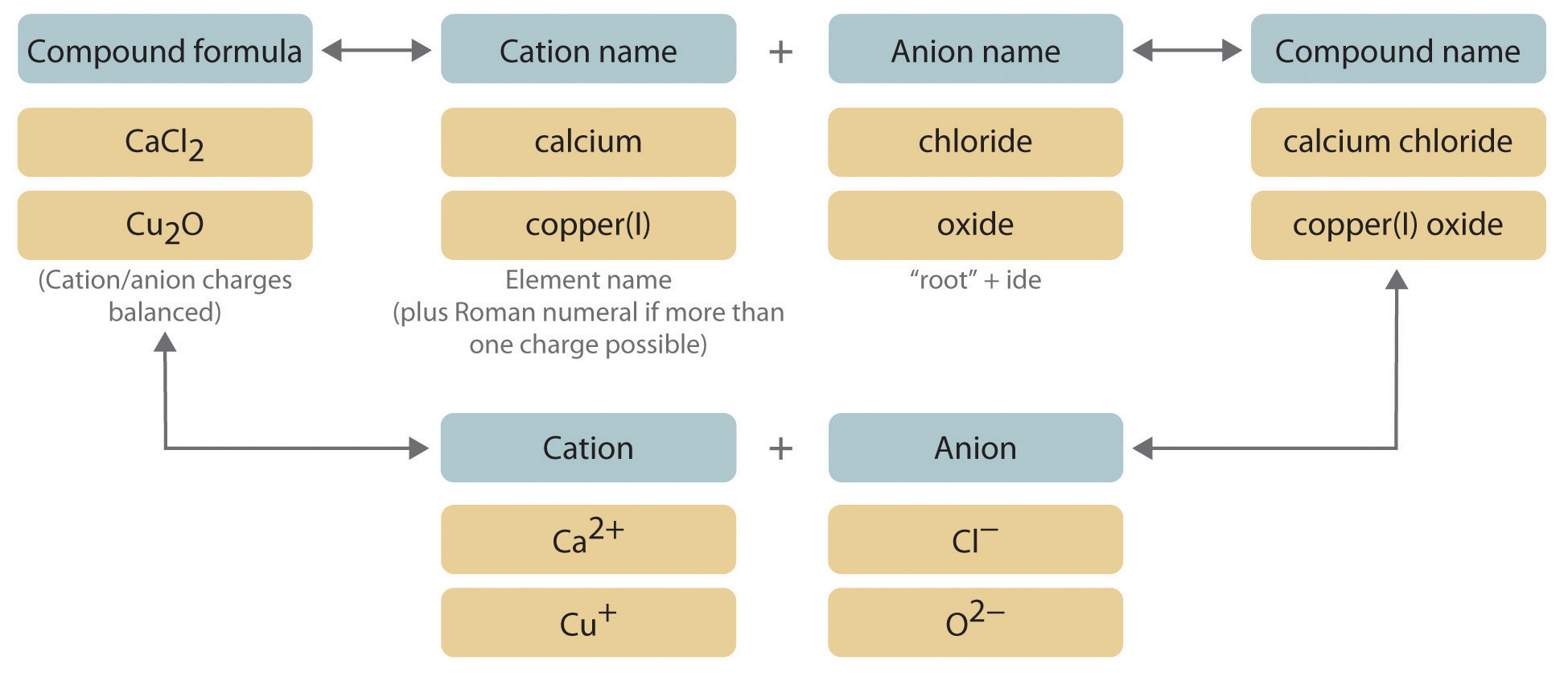
The full name of the cation is listed first. Then balance the ionic charges just as you would for any ionic compound. The number in.
A Guided lnqmry xercis Part I. Bases are named in the same way as other ionic compounds - the name of the cation is followed by the name of the anion To write the formula for a base first write the symbol for the metal cation followed by the formula for the hydroxide ion. The root of the anion name is listed second and is followed by the suffix ideAn anion is a negative ion.
Binary compounds of the elements with oxygen are generally named as element oxide with prefixes that indicate the number of atoms of each element per formula unit. Compounds between a metal and a nonmetal are ionic. When a metal and non-metal form an ionic molecule the metal will retain the element name and the non-metal will taken the suffix -ide.
Compound name cation name anion name Examples. Rules for Naming Binary Ionic Compounds Examples. Binary Ionic Compounds.
Ionic compounds are named as metal non-metalides for example. The only exception is binary compounds of oxygen with fluorine which are named as oxygen fluorides. Write the name of the compound.
Examples of binary compounds include sodium chloride NaCl and calcium oxide CaO. One example is MgCl 2 a coagulant used in the preparation of tofu from soybeans. This information is implied in the compounds name.
Na Cl- Ca 2 O 2- H Ionic Compounds with polyatomic ions. Learning Naming through Guided Inquiry Binary Ionic Compounds Compound Formula Metal Element Non-Metal Element Compound Name MgCl 2 magnesium chlorine magnesium chloride KBr potassium bromine potassium bromide Al 2 S 3 aluminum sulfur aluminum sulfide Ca 3 P 2 calcium phosphorous calcium phosphide Na 2 O sodium oxygen sodium oxide 1. Name of Cation metal Charge in parentheses Base Name of anion nonmetalide.
Na Sodium Mg 2 Magnesium Al 3 Aluminum. NAMING BINARY TERNARY AND QUATERNARY COMPOUNDS. NaCl sodium chloride BaF 2 barium fluoride CuO copper II oxide 1.
Sodium chloride NaCl magnesium oxide MgO That is the name of the metal followed by the name of the non-metal with its ending altered to be -ide. Cupric chloride CuCl cuprous chloride. Students often have difficulty remembering all the rules for naming ionic and covalent compounds.
Then either give the same or chemical formula as necessary for each problem below. Binary Molecular Compounds Binary molecular compounds that is covalent compounds that contain only two nonmetal elementsare named with a system utilizing Greek prefixes. Other elements such as arsenic sulfur polonium selenium and tellurium can also be considered.
When naming binary ionic compounds start with the cation specifying the charge if necessary then move on to the nonmetal anion element stem -ide. A cation is a positive ion. Binary compounds those containing only two elements are named in the following way.
Name the metal the cation as it appears on the Periodic Table. An ionic compound that contains only two elements one present as a cation and one as an anion is called a binary ionic compound An ionic compound that contains only two elements one present as a cation and one as an anion. L1 - 61 jj.
Format for naming Binary Compounds. How to Write Chemical Names for Binary Ionic Compounds Chemical name has 2 parts to represent both ions in the compound Step 1. CobaltIII oxide ironIII chloride copperII bromide lithium arsenide chromiumIII sulfide leadIV iodide potassium nitride ironII phosphide Use the rules you have determined to write the names of the following binary ionic compounds.
Both the cation and the anion in the compound are derived from a single element which has lost or gained one or more electrons. Nomenclature of Binary Ionic Compounds Composed of Maim group Elements Observing the Patterns. Metal nonmetal-ide where the metal the positively charged cation keeps its original name and the nonmetal the negatively charged anion takes on an -ide ending.
Name of Cation metal Base Name of anion nonmetal -ide Format For naming binary compounds with transitional metals. It is a group of binary compounds of hydrogen which includes a hydrogen atom attached to another atom which is in the 7th group of the periodic table. Formulas of Binary Ionic Compounds.
Binary Ionic Compounds formula contains one metal and one nonmetal. Name the positive ion write it in full usually a metal Step 2. CaCl 2 Calcium chlor ine Calcium chlor ide.
Pn IW r The Rules. First quickly scan the worksheet and circle any metals as a symbol or as a name that is a transition metal. In this context the -ide ending signifies a simple negative ion containing only.
Remember that transition metals can have multiple oxidation states so you are required. This set includes a bunch of binary compounds and it includes the compounds with transitional metals. The cation andor the anion is formed from a grouping of elements and that grouping is either positively or negatively charged.
Ionic compounds are formed when metals and nonmetals combine. MnF 2 Ni 3P 2 PbO 2 Cs 2S ScCl 3 MgI 2. The names of these compounds follow the pattern.
Name the negative ion do not capitalize Step 3. A monatomic meaning one-atom cation takes its name from the name of the element.
Ppt Nomenclature Binary Ionic Compounds Powerpoint Presentation Free Download Id 721132
5 7 Naming Ionic Compounds Chemistry Libretexts

Solved Part Ii Binary Ionic Compounds Containing Chegg Com

Forming And Naming Ionic Compounds Type 1 And 2 Binary Compounds Ppt Download



0 comments
Post a Comment